L’Oréal goes one step further in safety assessments by monitoring the potential adverse effects that may arise once the product is on the market through its international cosmeto-vigilance network. This network collects, validates and analyses, using recognised and rigorous methods, the adverse effects related to the use of a cosmetic product. This allows for appropriate corrective measures to be taken where necessary.
The safety cycle is summarised in the following graphic:
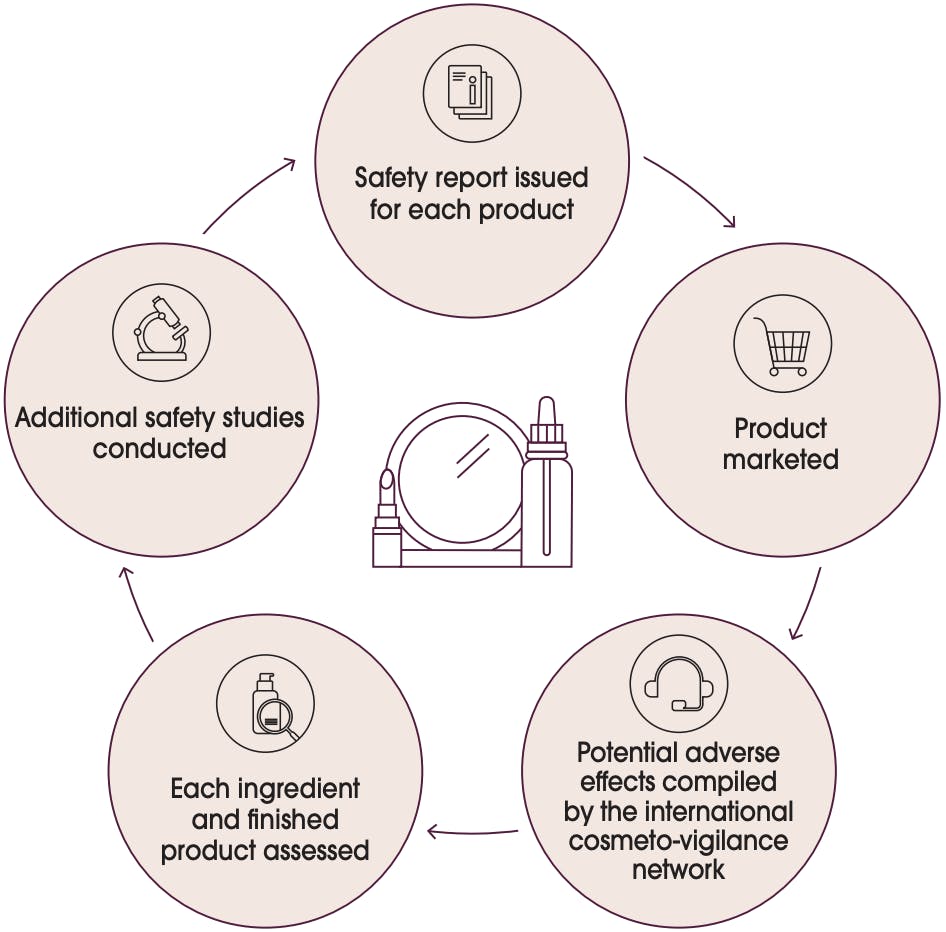
This graph shows the safety cycle summarised:
Safety report issued for each product
Product marketed
Potential adverse effects compiled by the international cosmeto-vigilance network
Each ingredient and finished product assessed
Additional safety studies conducted
In responding to questions that civil society may ask regarding certain substances and their effects on health and the environment, three points summarise L’Oréal’s position:
- vigilance with regard to any relevant new scientific data;
- cooperation with the relevant authorities; and
- precaution leading to the substitution of ingredients in the event of a proven or strongly suspected risk.
The launch of the website “Au coeur de nos produits” (Inside our products) in 2019 is testimony to the Group’s desire for increased transparency on this issue.
Finally, production quality standards define rules governing the quality of products, for all stages from creation to production and distribution. Almost all factories are ISO 9001 certified for their production and follow the Best Manufacturing Practices in accordance with the ISO 22716 standard.
The product safety assessment process
L’Oréal has set up a process to ensure that all products developed by the Group, whatever the geographic allocation of the laboratory in charge of the project, are subject to the same level of rigorous safety evaluation. The assessments by the Worldwide Safety Evaluation Department, based on a multidisciplinary scientific approach, are carried out at all stages of the life cycle of the products. This approach enables L’Oréal to meet the safety requirements of the national regulations in force in all the countries in which its products are put on sale, testifying to their safety of use. A safety assessment is conducted for each product launched on the market.
The product safety evaluation is based on the evaluation of each ingredient that enters into the composition of the product and the finished product itself. It is carried out on the basis of existing safety data and the latest scientific knowledge, and takes into consideration the conditions of use of the product. If necessary, L’Oréal conducts additional safety studies in qualified laboratories all over the world. The results of these studies are interpreted by experienced scientists who are specially trained in safety issues with regard to cosmetic ingredients and products.
Furthermore, L’Oréal’s ethical principles, rooted in both scientific rigour and responsiveness to societal concerns, lead to a pre-emptive approach whereby formulations are evolved by removing and/or replacing substances on the basis of new data.
L’Oréal’s added value, in terms of the safety assessment of ingredients and finished products, lies in its investment for nearly 40 years in the development of predictive methods and tissue engineering, and their international regulatory recognition. For many years, the Group has been investing in science and technology to create new evaluation tools which are used every day by safety assessors.
L’Oréal also works closely with all the international stakeholders involved in relevant industries in order to progress the development of alternative cross-disciplinary solutions in the field of safety assessment.
This longstanding commitment means that since 1989, or 14 years before regulations required, the Group no longer carries out animal testing in laboratories for any of its products. Equally, L’Oréal no longer tests its ingredients on animals. L’Oréal no longer tolerates any exceptions to this rule and this applies worldwide. The Group also does not delegate responsibility for doing so to anyone else. Some health authorities may nevertheless decide to carryout animal testing themselves for certain cosmetic products and this is still the case in China. For more than 10 years, L’Oréal has been the company most committed to getting Chinese authorities and scientists to recognise alternative methods and changing cosmetic regulations to achieve the complete and final elimination of animal testing. After the progress made in 2014, which put an end to the testing on animals of some products manufactured in China, since May 2021, all non-functional products imported into China no longer need to be tested on animals, provided that they are accompanied by a safety assessment and a manufacturing best practice certificate issued by their country of origin.
In fact, L’Oréal’s products continually evolve as and when technological innovations occur, but with the constant desire to guarantee the highest level of safety for both consumers and professionals.
